
We are advancing our drug candidate, pegozafermin, an investigational FGF21 analog with the potential to address a broad range of liver and cardiometabolic diseases, starting with MASH and SHTG.
Trial |
Indication |
Preclinical |
Phase 1 |
Phase 2 |
Phase 3 |
|---|---|---|---|---|---|
| MASH |
|
||||
| MASH | |||||
| SHTG | |||||
MASH, metabolic dysfunction-associated steatohepatitis; SHTG, severe hypertriglyceridemia.
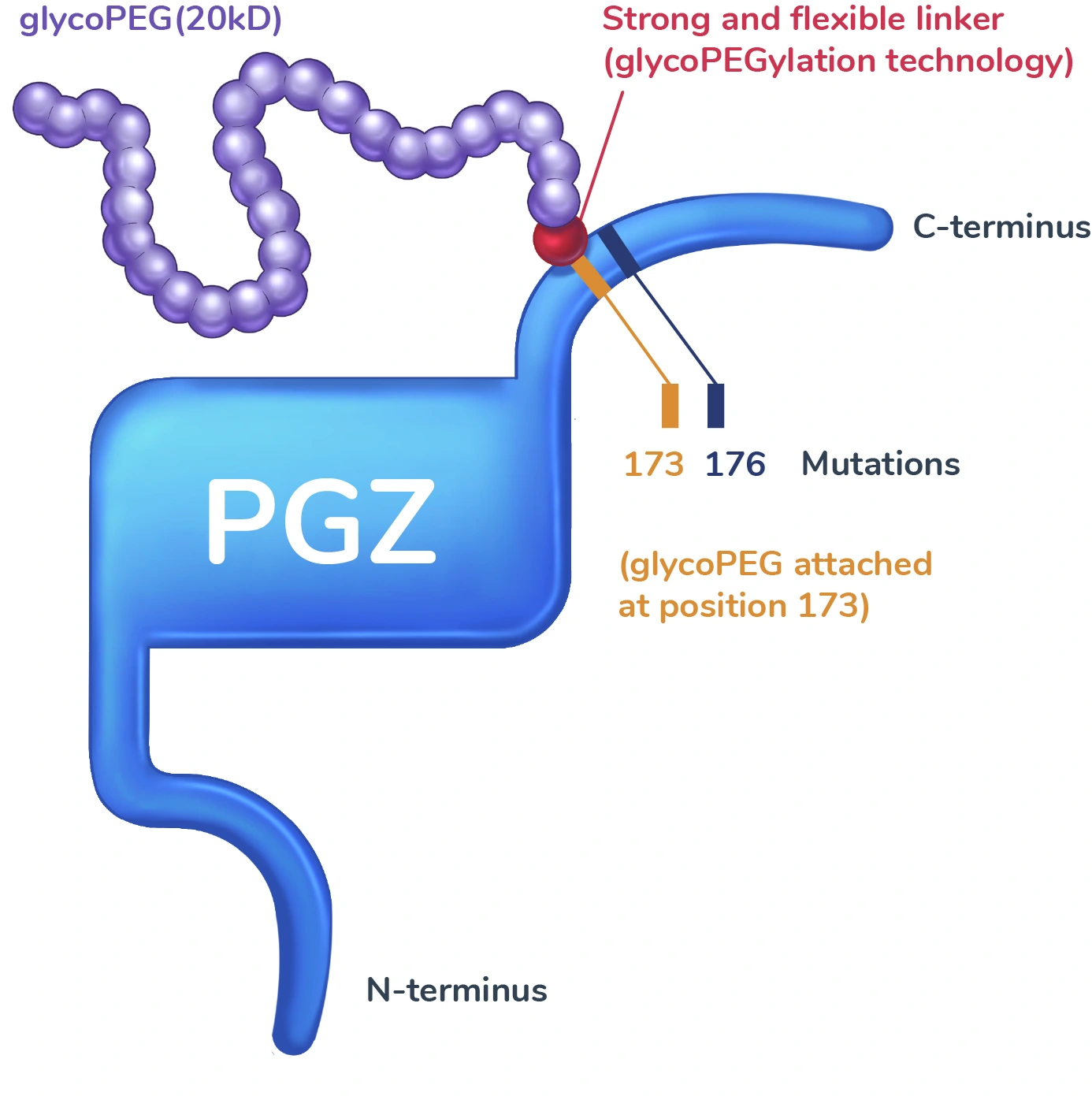
Pegozafermin is an investigational FGF21 analog engineered using glycoPEGylation technology with site-specific mutations designed to recapitulate the activity profile and prolong the half-life of the native FGF21 hormone, which may address underlying metabolic issues that drive liver and cardiometabolic diseases, such as MASH and SHTG.
Metabolic dysregulation can result in a multitude of liver and cardiometabolic disorders, including MASH and SHTG.
Fibroblast growth factor 21 (FGF21) is an endogenous hormone primarily secreted from the liver that acts as a master metabolic regulator with broad effects on energy expenditure and glucose and lipid metabolism. The effects of FGF21 are mediated through activation of its receptors (FGFR1, 2, or 3c) with co-receptor β-Klotho in tissues throughout the body, including the liver, adipose, pancreas, muscle, and brain.
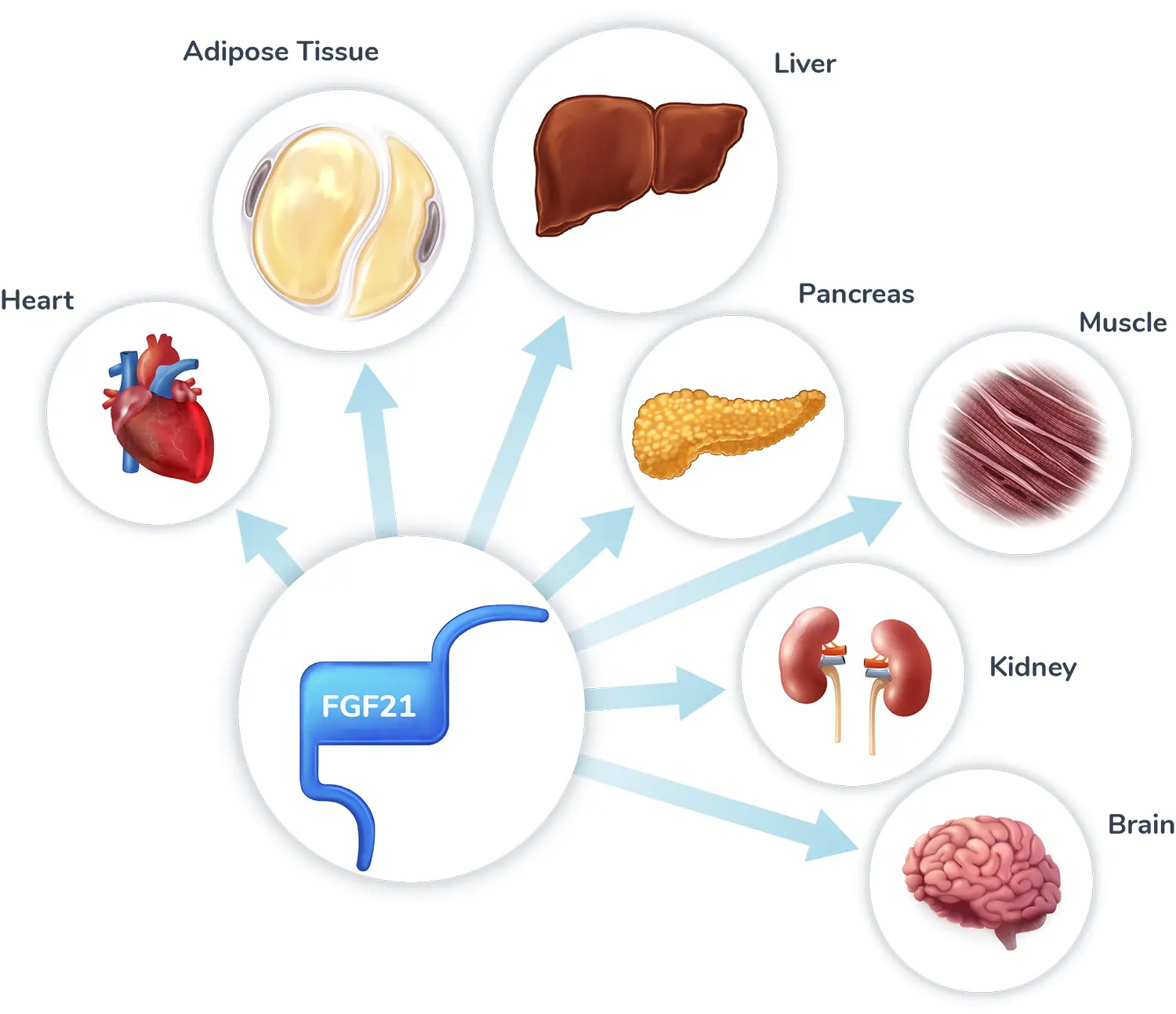
The role of FGF21 as a master metabolic regulator makes it a promising target for liver and cardiometabolic diseases; however, endogenous FGF21 has a short half-life, making it unsuitable for therapeutic use.
Pegozafermin is designed to overcome the short half-life of native FGF21 through two innovations: the addition of a tail with unique glycoPEGylation technology that has been shown to prolong its activity, and mutations at two sites that prevent its breakdown. We believe that pegozafermin recapitulates the activity of native FGF21 and therefore has the potential to help improve the underlying metabolic abnormalities that drive liver and cardiometabolic diseases.
We believe that the downstream effects of pegozafermin involve important metabolic pathways that reduce lipids, inhibit inflammation and fibrosis, improve insulin resistance, and reduce oxidative stress. One pathway helps to restore the balance of free fatty acid handling in the liver to reduce the fat accumulation, inflammation, and injury that drive fibrosis, which may lead to improvements in liver histology. Additional pathways involve increased production of adiponectin, a hormone secreted by adipose tissue that has insulin-sensitizing, anti-inflammatory, and anti-fibrotic effects.
In clinical trials, pegozafermin has demonstrated improvements in liver histology, liver fat reduction, and broad metabolic benefits in patients with MASH, as well as reduced triglycerides in patients with SHTG, thus addressing key drivers of morbidity and mortality in patients with cardiometabolic diseases.
Pegozafermin is an investigational FGF21 analog engineered with glycoPEGylation technology designed to recapitulate the activity profile and prolong the half-life of the native FGF21 hormone to address underlying metabolic issues that drive liver and cardiometabolic diseases, such as MASH and SHTG.
Randomized, controlled trial of the FGF21 analogue pegozafermin in NASH.
N Engl J Med 2023.
Pegozafermin for the treatment of non-alcoholic steatohepatitis patients with F2/F3 fibrosis: A multi-center, randomized, double-blind, placebo-controlled Phase 2b trial.
EASL Congress 2023.
The FGF21 analog pegozafermin in severe hypertriglyceridemia: A randomized phase 2 trial.
Nature Med 2023.
Pegozafermin inhibits MASH-induced hepatocellular carcinoma in the STAM™ mouse model.
EASL Congress 2023.
BIO89-100, a novel glycoPEGylated Fibroblast Growth Factor 21 (FGF21) analog activates FGF receptors (FGFR) 1c, 2c and 3c but not FGFR4 in L6 cells transfected with the four different human FGFRs and beta-Klotho (KLB).
AASLD 2020.
Weekly Subcutaneous Administration of BIO89-100, a Novel GlycoPEGylated-Fibroblast Growth Factor21 (FGF21) Analogue, Inhibits Sweetness Preference in Obese Cynomolgus Monkeys.
AASLD 2019.
1. Loomba R, Friedman SL, Shulman GI. Cell. 2021;184(10):2537-2564. 2. Raza Z, Mulki AK, Garufi LC, et al. J Fam Pract. 2020;69(4):180-187. 3. Sandesara PB, Virani SS, Fazio S, et al. Endocr Rev. 2019;40(2):537-557. 4. Loomba R, Lawitz EJ, Frias JP, et al. Lancet Gastroenterol Hepatol. 2023;8(2):120-132. 5. Fisher FM, Maratos-Flier E. Annu Rev Physiol. 2016;78:223-241. 6. Tillman EJ, Rolph T. Front Endocrinol (Lausanne). 2020;11:601290. 7. Markan KR, Naber MC, Ameka MK, et al. Diabetes. 2014;63(12):4057-4063. 8. Hotta Y, Nakamura H, Konishi M, et al. Endocrinology. 2009;150(10):4625-4633. 9. Coskun T, Bina HA, Schneider MA, et al. Endocrinology. 2008;149(12):6018-6027. 10. Kurosu H, Choi M, Ogawa Y, et al. J Biol Chem. 2007;282(37):26687-26695. 11. Rosenstock M. BIO89-100, a novel glycoPEGylated fibroblast growth factor 21 (FGF21) analog activated FGF receptors (FGFR) 1c, 2c and 3c but not FGFR4 in L6 cells transfected with the four different human FGFRs and beta-Kloto (KLB). Poster presented at: The Liver Meeting Digital Experience AASLD; November 13, 2020; Virtual. 12. BIO89-100 Phase 1b/2a Topline Results. Published online September 14, 2020. Accessed March 13, 2023. https://ir.89bio.com/static-files/0da30221-2ed4-4ada-b666-1c34e400b6c2. 13. Kharitonenkov A, Shiyanova TL, Koester A, et al. J Clin Invest. 2005;115(6):1627-1635. 14. Kharitonenkov A, Wroblewski VJ, Koester A, et al. Endocrinology. 2007;148(2):774-781. 15. Loomba R. BIO89-100, a novel glycopegylated FGF21 analogue, demonstrates robust reduction in serum lipids and long half-life in a phase 1 randomized, controlled single ascending dose trial in healthy subjects. Presented at: The American Association for the Study of Liver Diseases (AASLD) Meeting; November 8, 2019; Boston, MA. 16. 89bio. Developing a differentiated FGF21 for NASH and SHTG. Corporate Presentation. Published online January 2020. 17. Zhang F, Yu L, Lin X, et al. Mol Endocrinol. 2015;29(10):1400-1413. 18. Lin Z, Tian H, Lam KSL, et al. Cell Metab. 2013;17(5):779-789. 19. 89bio. Pegozafermin phase 2b (ENLIVEN) topline results in nonalcoholic steatohepatitis (NASH). Corporate Presentation. Published online March 2023. 20. Loomba R. Pegozfermin led to improved liver histology, liver-related non-invasive tests and metabolic profile, with favorable safety and tolerability, in an open-label cohort of a Phase 1b/2a study in subjects with non-alcoholic steatohepatitis. Poster presented at: EASL International Liver Congress; June 2022; London. 21. Handy JA, Fu PP, Kumar P, et al. Biochem J. 2011;440(3):385-395. 22. Bhatt DL, Bays HE, Miller M, et al. Nat Med. Published online June 24, 2023:1-11.